Multiple Choice
What is ΔG° at 298 K for the following reaction? (F = 96,500 C • mol -1) 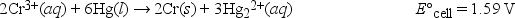
A) -921 kJ/mol
B) -767 kJ/mol
C) -460 kJ/mol
D) -307 kJ/mol
E) -1840 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: At equilibrium E° = 0.
Q20: What is the equilibrium constant at 25°C
Q21: Which component of the following cell is
Q22: A voltaic cell is prepared using copper
Q23: Lithium-ion batteries can be recharged many times.
Q25: What mass of nickel may be electroplated
Q26: A voltaic cell consists of a Hg/Hg<sub>2</sub><sup>2+</sup>
Q27: What is E°<sub>cell</sub> for the following reaction,
Q29: If the following electrochemical cell is constructed,
Q148: Iron objects such as storage tanks and