Multiple Choice
If the following electrochemical cell is constructed, what voltage will be measured on the voltmeter? 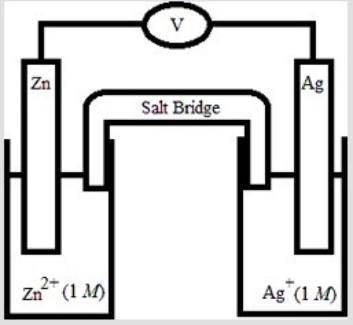
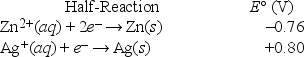
A) 2.36 V
B) 1.56 V
C) 0.04 V
D) -0.04 V
E) -2.36 V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q24: What is ΔG° at 298 K for
Q25: What mass of nickel may be electroplated
Q26: A voltaic cell consists of a Hg/Hg<sub>2</sub><sup>2+</sup>
Q27: What is E°<sub>cell</sub> for the following reaction,
Q30: Based on the data presented below, which
Q31: What is the cell potential at 25°C
Q32: Reduction occurs at the anode of a
Q33: Given Cu<sup>2+</sup>(aq) + 2e<sup>-</sup> → Cu(s) E°
Q34: If E° for X + e<sup>-</sup> →
Q148: Iron objects such as storage tanks and