Multiple Choice
Based on the following electrochemical cell, what is the standard reduction potential of metal M at 298 K? 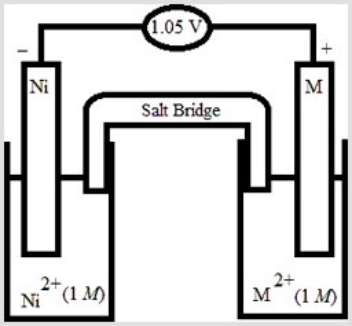
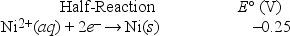
A) -1.40 V
B) -0.80 V
C) +0.25 V
D) +0.80 V
E) +1.40 V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q112: The voltaic cell composed of Co(s), Co<sup>2+</sup>(aq),
Q113: Electrons flow to the cathode in a
Q114: What is E°<sub>cell</sub> for a galvanic cell
Q115: What quantity of charge is required to
Q116: Which is the correct cell diagram for
Q117: What is ΔG° at 200°C for the
Q118: What mass of oxygen gas is produced
Q119: _ is the process that uses electrical
Q120: What is the name given to the
Q122: Which one of the following statements relating