Multiple Choice
Based on the following electrochemical cell, what is the standard reduction potential of metal M at 298 K? (R = 8.314 J/K • mol, F = 96500 C/mol) 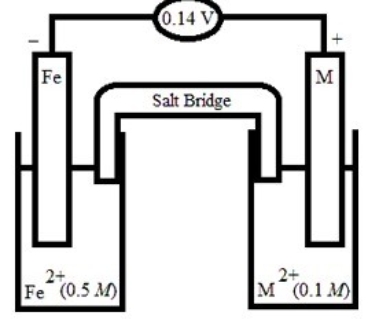
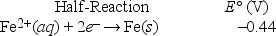
A) -0.54 V
B) +0.60 V
C) -0.30 V
D) +0.56 V
E) -0.28 V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q77: Complete and balance the following redox equation.
Q78: What is the name given to the
Q79: When the following redox equation is balanced
Q80: What is the name given to the
Q81: Which is the half-reaction at the anode
Q83: When the following equation is balanced with
Q84: Given the following standard reduction potentials in
Q85: What is the purpose of a salt
Q86: When the following redox equation is balanced
Q87: A certain electrochemical cell has for its