Short Answer
Given the following standard reduction potentials in acid solution 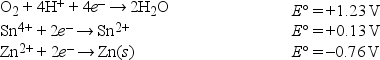 write the formula of the strongest reducing agent.
write the formula of the strongest reducing agent.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q79: When the following redox equation is balanced
Q80: What is the name given to the
Q81: Which is the half-reaction at the anode
Q82: Based on the following electrochemical cell, what
Q83: When the following equation is balanced with
Q85: What is the purpose of a salt
Q86: When the following redox equation is balanced
Q87: A certain electrochemical cell has for its
Q88: _ occurs at the cathode in a
Q89: What is the half-reaction that occurs at