Multiple Choice
What is ΔG°rxn at 298 K for the following reaction? 2Cl2(g) + SO2(g) → SOCl2(g) + Cl2O(g) 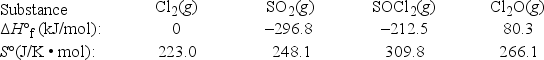
A) 129.3 kJ/mol
B) 133.4 kJ/mol
C) 196.0 kJ/mol
D) 199.8 kJ/mol
E) 229.6 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q74: At equilibrium, ΔG° = 0.
Q75: _ is the pressure required for standard
Q76: The following reaction is spontaneous under standard
Q77: Consider the figure below which shows ΔG°
Q78: Which of the following has ΔG°<sub>f </sub>=
Q80: Which of the following is an example
Q81: For the reaction of xenon and fluorine
Q82: Which of the following substances has the
Q83: Which statement is correct?<br>A) Heating always decreases
Q84: Which of the following will decrease the