Multiple Choice
Consider the figure below which shows ΔG° for a chemical process plotted against absolute temperature. Which one of the following is an incorrect conclusion, based on the information in the diagram? 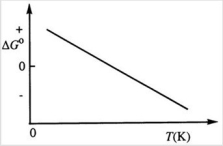
A) ΔH° > 0.
B) ΔS° > 0.
C) The reaction is spontaneous at high temperatures.
D) ΔS° increases with temperature while ΔH° remains constant.
E) There exists a certain temperature at which ΔH° = TΔS°.
Correct Answer:

Verified
Correct Answer:
Verified
Q39: The higher the pressure of a gas
Q73: The entropy change ΔS° at 298 K
Q74: At equilibrium, ΔG° = 0.
Q75: _ is the pressure required for standard
Q76: The following reaction is spontaneous under standard
Q78: Which of the following has ΔG°<sub>f </sub>=
Q79: What is ΔG°<sub>rxn </sub>at 298 K for
Q80: Which of the following is an example
Q81: For the reaction of xenon and fluorine
Q82: Which of the following substances has the