Multiple Choice
Calculate ΔG°rxn for the following reaction of ammonia with fluorine. 2NH3(g) + 5F2(g) → N2F4(g) + 6HF(g) 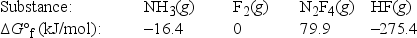
A) 179.1 kJ/mol
B) -179.1 kJ/mol
C) 1539.7 kJ/mol
D) -1539.7 kJ/mol
E) -211.9 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: a. Explain what is meant by a
Q98: Calculate ΔG° for the combustion of ethanol
Q99: Which, if any, of the following processes
Q100: Which is true for a system at
Q101: Which is necessary for a process to
Q102: When a sky diver free-falls through the
Q103: The temperature at which the following process
Q104: Which is always true for an exothermic
Q105: Which response includes all of the following
Q107: ΔS<sub>univ</sub> = -1 for a spontaneous reaction.