Multiple Choice
The temperature at which the following process reaches equilibrium at 1.0 atm is the normal boiling point of hydrogen peroxide. (R = 8.314 J/K • mol) H2O2(l)  H2O2(g)
H2O2(g)
Use the following thermodynamic information at 298 K to estimate this temperature. 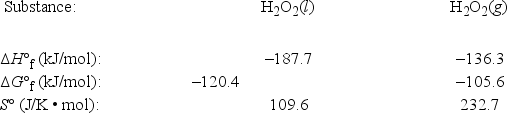
A) 120°C
B) 144°C
C) 196°C
D) 418°C
E) 585°C
Correct Answer:

Verified
Correct Answer:
Verified
Q2: a. Explain what is meant by a
Q98: Calculate ΔG° for the combustion of ethanol
Q99: Which, if any, of the following processes
Q100: Which is true for a system at
Q101: Which is necessary for a process to
Q102: When a sky diver free-falls through the
Q104: Which is always true for an exothermic
Q105: Which response includes all of the following
Q106: Calculate ΔG°<sub>rxn</sub> for the following reaction of
Q107: ΔS<sub>univ</sub> = -1 for a spontaneous reaction.