Multiple Choice
Below is a representation of the sparingly soluble salt MX at equilibrium in aqueous solution. (Each circle represents 1.0 ×10-3 mol of atoms, and the volume of the box is 1.0 L. Solvent water molecules are omitted for clarity.) 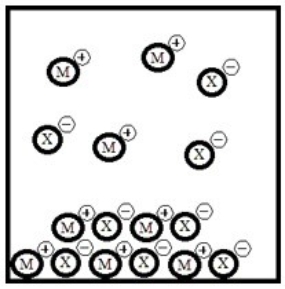 What is Ksp for MX?
What is Ksp for MX?
A) 1.5 ×10-5
B) 6.0 ×10-6
C) 3.6 ×10-1
D) 1.8 ×10-3
E) 9.0 ×10-6
Correct Answer:

Verified
Correct Answer:
Verified
Q60: Increasing the concentrations of the components of
Q63: When a weak acid is titrated with
Q64: Calculate the pH of a buffer solution
Q65: The _ is the solution that is
Q66: Suppose 50.0 mL of a 0.100 M
Q67: Consider the dissolution of MnS in water.
Q70: For the 1:1 ratio combination of a
Q71: What is the Henderson-Hasselbach equation?<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8482/.jpg"
Q72: A 50.00-mL solution of 0.10 M HNO<sub>2</sub>
Q110: What is the pH at the equivalence