Multiple Choice
Suppose 50.0 mL of a 0.100 M solution of the weak acid HA is titrated with a 0.100 M solution of NaOH. Which diagram best represents the equilibrium composition of the solution once 25.0 mL of titrant have been added? (Solvent water molecules are omitted for clarity.)
A) 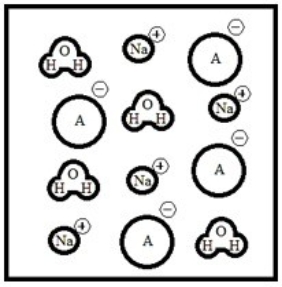
B) 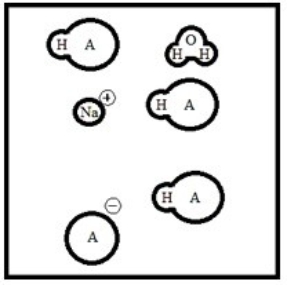
C) 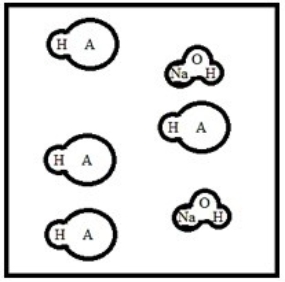
D) 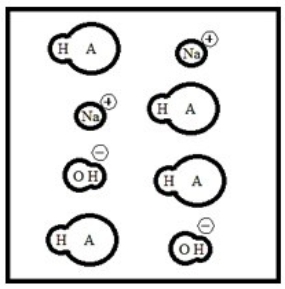
E) 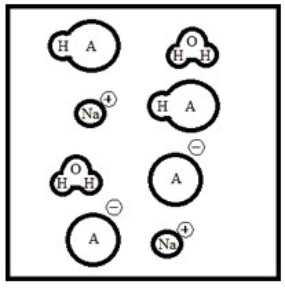
Correct Answer:

Verified
Correct Answer:
Verified
Q61: What is the effect on equilibrium when
Q62: What is the pH of a solution
Q63: When a weak acid is titrated with
Q64: Calculate the pH of a buffer solution
Q65: The _ is the solution that is
Q67: Consider the dissolution of MnS in water.
Q68: Below is a representation of the sparingly
Q70: For the 1:1 ratio combination of a
Q71: What is the Henderson-Hasselbach equation?<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8482/.jpg"
Q110: What is the pH at the equivalence