Multiple Choice
At what temperature is KP = 4.00 for the reaction N2O4(g)  2NO2(g) ?
2NO2(g) ? 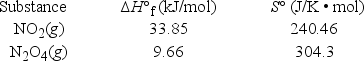 (R = 8.314 J/K • mol)
(R = 8.314 J/K • mol)
A) 197°C
B) 56°C
C) 36°C
D) 79°C
E) 476°C
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q34: Consider the reaction N<sub>2</sub>(g) + 3H<sub>2</sub>(g) <img
Q35: Consider the reaction N<sub>2</sub>(g) + O<sub>2</sub>(g) <img
Q36: The following reactions occur at 500 K.
Q37: A container was charged with hydrogen, nitrogen,
Q38: At 400ºC, K<sub>c</sub> = 64 for the
Q40: Which equation is correct?<br>A) ΔG = ΔG°
Q42: At 25°C, the equilibrium constant, K<sub>c</sub>, for
Q43: At 450°C, tert-butyl alcohol decomposes into water
Q44: What is used to explain the effect
Q132: At equilibrium, the rate of the forward