Multiple Choice
The following reactions occur at 500 K. Arrange them in order of increasing tendency to proceed to completion (least completion → greatest completion) . 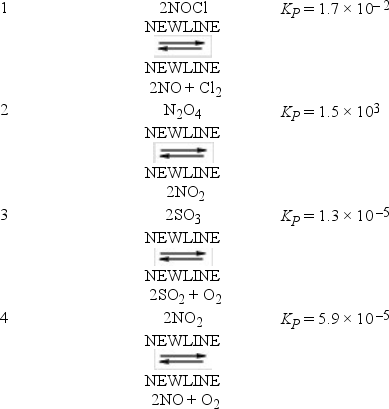
A) 2 < 1 < 3 < 4
B) 3 < 1 < 4 < 2
C) 3 < 4 < 1 < 2
D) 4 < 3 < 2 < 1
E) 4 < 3 < 1 < 2
Correct Answer:

Verified
Correct Answer:
Verified
Q31: When a reaction reaches _, the concentration
Q32: Changing the amount of reactant or product
Q33: The reaction 2H<sub>2</sub>O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8482/.jpg" alt="The reaction
Q34: Consider the reaction N<sub>2</sub>(g) + 3H<sub>2</sub>(g) <img
Q35: Consider the reaction N<sub>2</sub>(g) + O<sub>2</sub>(g) <img
Q37: A container was charged with hydrogen, nitrogen,
Q38: At 400ºC, K<sub>c</sub> = 64 for the
Q39: At what temperature is K<sub>P</sub> = 4.00
Q40: Which equation is correct?<br>A) ΔG = ΔG°
Q132: At equilibrium, the rate of the forward