Multiple Choice
Hydrogen sulfide can be formed in the following reaction: H2(g) + 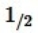 S2(g)
S2(g)  H2S(g) ; ΔH°rxn = -92 kJ/mol
H2S(g) ; ΔH°rxn = -92 kJ/mol
The equilibrium constant,KP,is 106 at 1023 K. Estimate the value of KP at 1218 K. (R = 8.314 J/K • mol)
A) 5.05
B) 18.8
C) 34.7
D) 88.9
E) 598
Correct Answer:

Verified
Correct Answer:
Verified
Q40: For any reaction, if ΔG° > 0,
Q55: For the endothermic reaction A<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8482/.jpg"
Q56: At elevated temperatures, hydrogen iodide may decompose
Q57: The reaction system POBr<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8482/.jpg" alt="The
Q58: The equilibrium constant for the reaction AgBr(s)
Q59: The reaction system POBr<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8482/.jpg" alt="The
Q61: For the following reaction at 25ºC, is
Q62: Which statement is correct?<br>A) If Q <
Q63: The equilibrium constants (expressed in atm) for
Q64: For the reaction SO<sub>2</sub>(g) + NO<sub>2</sub>(g) <img