Multiple Choice
For the endothermic reaction A2(g)  2A(g) , a snapshot of an equilibrium mixture of A(g) and A2(g) may look as follows. (Each circle represents 1.0 mol of A atoms, and the volume of the box is 1.0 L.)
2A(g) , a snapshot of an equilibrium mixture of A(g) and A2(g) may look as follows. (Each circle represents 1.0 mol of A atoms, and the volume of the box is 1.0 L.) 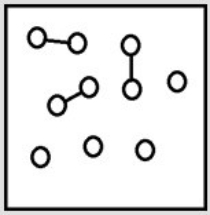 What is the equilibrium constant KP for this reaction at 298 K? (R = 0.08206 L • atm/K • mol)
What is the equilibrium constant KP for this reaction at 298 K? (R = 0.08206 L • atm/K • mol)
A) 1.3 ×102
B) 65
C) 0.22
D) 16
E) 5.33
Correct Answer:

Verified
Correct Answer:
Verified
Q50: At 850°C, the equilibrium constant, K<sub>P</sub>, for
Q51: Based on the following data, what is
Q52: Carbon tetrachloride reacts at high temperatures with
Q53: Which of the following is the expression
Q54: Suppose 50.0 g of N<sub>2</sub>O<sub>4</sub> is introduced
Q56: At elevated temperatures, hydrogen iodide may decompose
Q57: The reaction system POBr<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8482/.jpg" alt="The
Q58: The equilibrium constant for the reaction AgBr(s)
Q59: The reaction system POBr<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8482/.jpg" alt="The
Q60: Hydrogen sulfide can be formed in the