Multiple Choice
A study of the decomposition reaction 3RX2 → 3R + 6X yields the following initial rates. 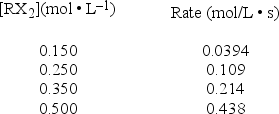 What is the rate constant for the reaction?
What is the rate constant for the reaction?
A) 0.0103 L • mol-1 • s-1
B) 0.263 L • mol-1 • s-1
C) 0.571 L • mol-1 • s-1
D) 1.17 L • mol-1 • s-1
E) 1.75 L • mol-1 • s-1
Correct Answer:

Verified
Correct Answer:
Verified
Q20: The elementary step of the reaction mechanism
Q21: A first-order reaction has a rate constant
Q22: Consider the following reaction 8A(g) + 5B(g)
Q23: Consider reactions A, B, and C, which
Q26: Consider the general gas-phase reaction of a
Q27: A certain reaction A → products is
Q28: The following diagram represents the first-order decomposition
Q29: A rate constant obeys the Arrhenius equation,
Q30: What is the integrated rate law for
Q77: The rate law cannot be predicted from