Multiple Choice
Consider reactions A, B, and C, which have the potential energy profiles shown. Assuming that the reactions have roughly the same frequency factors, which reaction is the slowest? 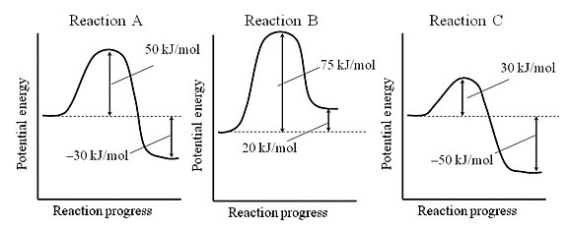
A) Reaction A
B) Reaction B
C) Reaction C
D) All of the reactions have the same rate.
Correct Answer:

Verified
Correct Answer:
Verified
Q18: The oxidation of iodide ions by arsenic
Q19: The rate law for the rearrangement of
Q20: The elementary step of the reaction mechanism
Q21: A first-order reaction has a rate constant
Q22: Consider the following reaction 8A(g) + 5B(g)
Q25: A study of the decomposition reaction 3RX<sub>2</sub>
Q26: Consider the general gas-phase reaction of a
Q27: A certain reaction A → products is
Q28: The following diagram represents the first-order decomposition
Q77: The rate law cannot be predicted from