Multiple Choice
Consider the following potential energy profile for the A → B reaction. How many intermediates are formed? 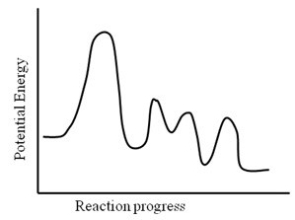
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q32: The reaction CH<sub>3</sub>NC(g) → CH<sub>3</sub>CN(g) is first-order
Q54: According to the collision theory of reaction
Q110: The elementary reaction HBr(g) + Br(g) →
Q111: A catalyst increases the rate of the
Q112: A reactant R is being consumed in
Q113: What is the activation energy for a
Q114: For the reaction C<sub>6</sub>H<sub>14</sub>(g) → C<sub>6</sub>H<sub>6</sub>(g) +
Q117: The following diagram represents the second-order decomposition
Q118: A first-order reaction has a rate constant
Q120: Consider the following potential energy profile for