Multiple Choice
Consider the following potential energy profile for the A → B reaction. Assuming that the frequency factor for the reaction is 5.5 × 1010 s-1, what is the rate constant of this first-order reaction at 310 K? 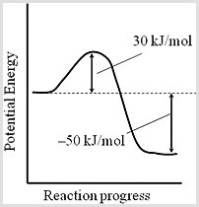
A) 2 × 102 s-1
B) 5 × 105 s-1
C) 1 × 1019 s-1
D) 2 × 107 s-1
Correct Answer:

Verified
Correct Answer:
Verified
Q32: The reaction CH<sub>3</sub>NC(g) → CH<sub>3</sub>CN(g) is first-order
Q54: According to the collision theory of reaction
Q115: Consider the following potential energy profile for
Q117: The following diagram represents the second-order decomposition
Q118: A first-order reaction has a rate constant
Q121: For the reaction A(g) + 2B(g) →
Q122: A certain first-order reaction A → B
Q123: Consider the general reaction 5Br<sup>-</sup>(aq) + BrO<sub>3</sub><sup>-</sup>(aq)
Q124: The following is an Arrhenius plot of
Q125: For the reaction A + 2B →