Multiple Choice
Below is a plot of the reaction of A decomposing to form products. 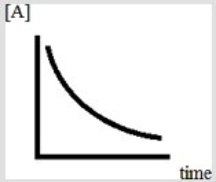 Based on the figure, which might be the order of the reaction with respect to A?
Based on the figure, which might be the order of the reaction with respect to A?
A) The reaction must only be zeroth order in A.
B) The reaction must only be first order in A.
C) The reaction must only be second order in A.
D) The reaction could be either first order in A or second order in A.
E) The reaction could be of any order in A.
Correct Answer:

Verified
Correct Answer:
Verified
Q122: A certain first-order reaction A → B
Q123: Consider the general reaction 5Br<sup>-</sup>(aq) + BrO<sub>3</sub><sup>-</sup>(aq)
Q124: The following is an Arrhenius plot of
Q125: For the reaction A + 2B →
Q126: Which is the correct unit for a
Q127: The rate law predicted by the following
Q128: The step of the reaction mechanism which
Q129: What is the name given to the
Q131: The reaction A + 2B → Products
Q132: The following initial rate data apply to