Multiple Choice
The following is an Arrhenius plot of a first-order reaction. The rate constant is measured in units of s-1. 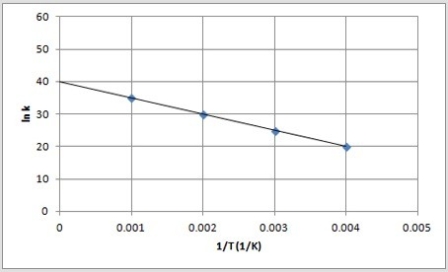 If a catalyst is added to the reaction, which could correspond to an Arrhenius plot of the catalyzed reaction?
If a catalyst is added to the reaction, which could correspond to an Arrhenius plot of the catalyzed reaction?
A) 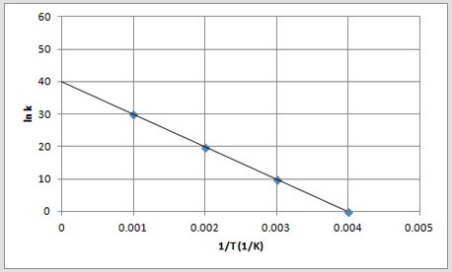
B) 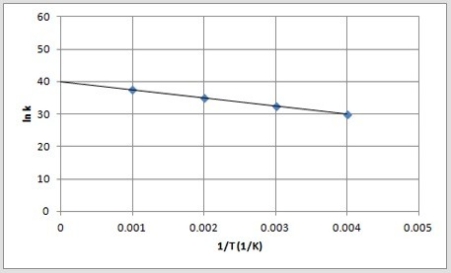
C) 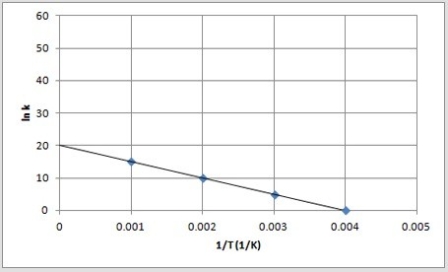
D) 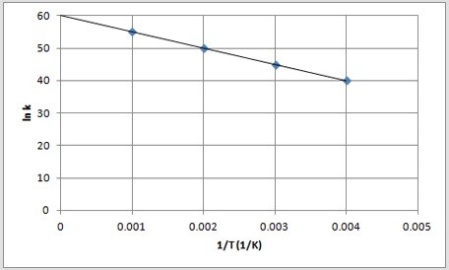
E) 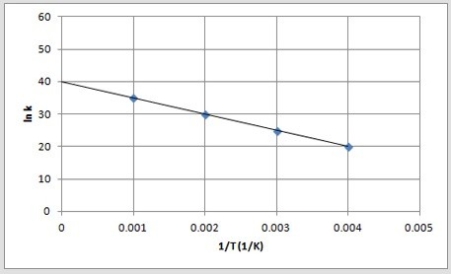
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q54: According to the collision theory of reaction
Q120: Consider the following potential energy profile for
Q121: For the reaction A(g) + 2B(g) →
Q122: A certain first-order reaction A → B
Q123: Consider the general reaction 5Br<sup>-</sup>(aq) + BrO<sub>3</sub><sup>-</sup>(aq)
Q125: For the reaction A + 2B →
Q126: Which is the correct unit for a
Q127: The rate law predicted by the following
Q128: The step of the reaction mechanism which
Q129: What is the name given to the