Multiple Choice
The following is an Arrhenius plot of a first-order reaction. The rate constant is measured in units of s-1. 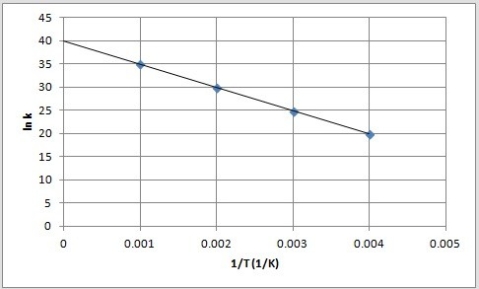 Based on this Arrhenius plot, what is the activation energy of the reaction? (R = 8.314 J/K •mol)
Based on this Arrhenius plot, what is the activation energy of the reaction? (R = 8.314 J/K •mol)
A) 4.0 kJ/mol
B) 5.0 kJ/mol
C) 40 kJ/mol
D) 42 kJ/mol
E) 50 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q25: A reaction intermediate is a species corresponding
Q45: The rate law for the reaction H<sub>2</sub>O<sub>2</sub>
Q46: What is the half-life for a second-order
Q47: What is the slope of a plot
Q48: The rate constant for a reaction is
Q49: What is the name given to the
Q51: The isomerization of methyl isocyanide, CH<sub>3</sub>NC →
Q53: For the overall chemical reaction shown below,
Q54: The _ is the equation relating the
Q55: For the following reaction, ΔP(C<sub>6</sub>H<sub>14</sub>)/Δt was found