Multiple Choice
Select the pair of substances in which the one with the lower vapor pressure at a given temperature is listed first.
A) 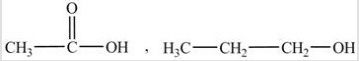
B) PH3, NH3
C) CF4, CBr4
D) C3H8, C4H10
E) F2, Cl2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q71: Select the pair of substances in which
Q93: Octane, C<sub>8</sub>H<sub>18</sub>, boils at 125°C, whereas water
Q94: _ _ are the attractions that hold
Q95: If a molecule at the surface of
Q96: Below is a representation of liquid water
Q97: The triple point of iodine is at
Q99: What is the attractive force between like
Q100: Which would be expected to have the
Q101: Octane has a vapor pressure of 40.
Q146: Identify the dominant (strongest)type of intermolecular force