Multiple Choice
Below is a representation of liquid water in equilibrium with its water vapor in a rigid container at 35ºC. The circles represent water vapor. 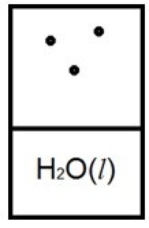 Which diagram below best represents liquid water in equilibrium with its water vapor at 70ºC? The heat of vaporization of water is 40.7 kJ/mol. (R = 8.314 J/K • mol)
Which diagram below best represents liquid water in equilibrium with its water vapor at 70ºC? The heat of vaporization of water is 40.7 kJ/mol. (R = 8.314 J/K • mol)
A) 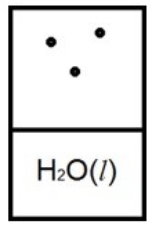
B) 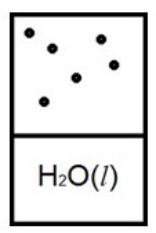
C) 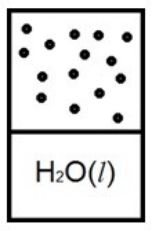
D) 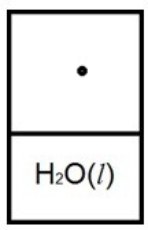
E) 
Correct Answer:

Verified
Correct Answer:
Verified
Q91: What is the intermolecular force that exists
Q92: Which of the following pure substances has
Q93: Octane, C<sub>8</sub>H<sub>18</sub>, boils at 125°C, whereas water
Q94: _ _ are the attractions that hold
Q95: If a molecule at the surface of
Q97: The triple point of iodine is at
Q98: Select the pair of substances in which
Q99: What is the attractive force between like
Q100: Which would be expected to have the
Q101: Octane has a vapor pressure of 40.