Multiple Choice
What is ΔH°rxn for the following reaction? NO2(g) + CO(g) → CO2(g) + NO(g) 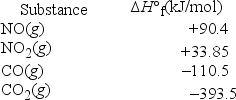
A) 339.6 kJ
B) 379.8 kJ
C) -226.5 kJ
D) -339.6 kJ
E) -379.8 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q26: Cold packs, whose temperatures are lowered when
Q39: Clearly state the thermodynamic standard state of<br>a.
Q125: How much heat is required to raise
Q126: What is ΔH°<sub>rxn</sub> for the following reaction?
Q129: If two solutions are mixed together in
Q131: The standard temperature is _; the standard
Q132: A glass containing 200.0 g of H<sub>2</sub>O
Q133: A 307-g sample of an unknown mineral
Q134: A system contracts from an initial volume
Q135: Suppose a new metallic element X is