Multiple Choice
Suppose a new metallic element X is discovered, and its reactions with oxygen gas and chlorine gas at 298 K are studied. 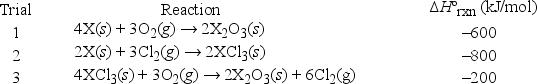 However, it is later discovered that one of the samples was contaminated, and the ΔHºrxn value from this trial is not reliable. Which trial had the contaminated sample, and what should the correct value of ΔHºrxn be for this trial?
However, it is later discovered that one of the samples was contaminated, and the ΔHºrxn value from this trial is not reliable. Which trial had the contaminated sample, and what should the correct value of ΔHºrxn be for this trial?
A) Trial 1 had the contaminated sample; its ΔHºrxn value should be -900 kJ/mol.
B) Trial 2 had the contaminated sample; its ΔHºrxn value should be +100 kJ/mol.
C) Trial 3 had the contaminated sample; its ΔHºrxn value should be -1000 kJ/mol.
D) Trial 3 had the contaminated sample; its ΔHºrxn value should be +1000 kJ/mol.
E) Not enough information is provided; all three trials must be redone.
Correct Answer:

Verified
Correct Answer:
Verified
Q130: What is ΔH°<sub>rxn </sub>for the following reaction?
Q131: The standard temperature is _; the standard
Q132: A glass containing 200.0 g of H<sub>2</sub>O
Q133: A 307-g sample of an unknown mineral
Q134: A system contracts from an initial volume
Q136: Given that CaO(s) + H<sub>2</sub>O(l) → Ca(OH)<sub>2</sub>(s),
Q137: For which of the substances below is
Q138: Pentaborane B<sub>5</sub>H<sub>9</sub>(s) burns vigorously in O<sub>2</sub> to
Q139: When heat is absorbed by the system
Q140: Atoms A and Z may form either