Multiple Choice
The bond enthalpy of the Br-Cl bond is equal to ΔH°rxn for the following reaction. BrCl(g) → Br(g) + Cl(g)
Using the following data, what is the bond enthalpy of the Br-Cl bond? 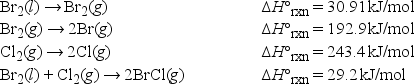
A) 219.0 kJ/mol
B) 203.5 kJ/mol
C) 14.6 kJ/mol
D) 438.0 kJ/mol
E) 407.0 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q39: The sign of w when work is
Q40: A system expands from a volume of
Q41: A student used a bomb calorimeter to
Q42: A system initially has an internal energy
Q44: A system that does no work but
Q45: Styrene, C<sub>8</sub>H<sub>8</sub>, is one of the substances
Q46: An important step in the synthesis of
Q47: The specific heat (capacity) is<br>A) the amount
Q48: A Snickers® candy bar contains 280 Calories,
Q100: A home aquarium is an example of