Multiple Choice
An important step in the synthesis of nitric acid is the conversion of ammonia to nitric oxide according to the following balanced chemical equation. What is ΔH°rxn for this reaction? 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g) 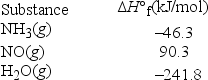
A) -1274.8 kJ/mol
B) -904.4 kJ/mol
C) -240.2 kJ/mol
D) -197.8kJ
E) 197.8 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q41: A student used a bomb calorimeter to
Q42: A system initially has an internal energy
Q43: The bond enthalpy of the Br-Cl bond
Q44: A system that does no work but
Q45: Styrene, C<sub>8</sub>H<sub>8</sub>, is one of the substances
Q47: The specific heat (capacity) is<br>A) the amount
Q47: An exothermic reaction causes the surroundings to<br>A)warm
Q48: A Snickers® candy bar contains 280 Calories,
Q49: A system absorbs 21.6 kJ of heat
Q50: The sign of q when heat is