Multiple Choice
Atoms A and Z may form either single bonds or double bonds. Shown is an energy diagram for the formation of a single bond between A and Z from the individual atoms in the gas phase. 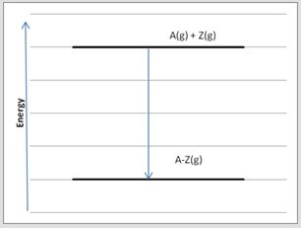 Which diagram below corresponds to the reaction A(g) + Z(g) → A=Z(g) ?
Which diagram below corresponds to the reaction A(g) + Z(g) → A=Z(g) ?
A) 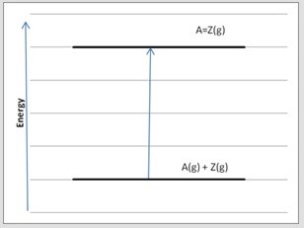
B) 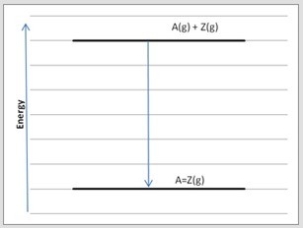
C) 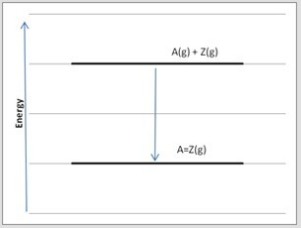
D) 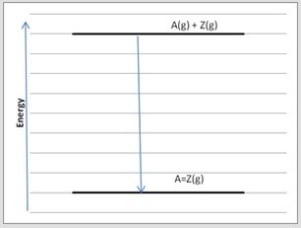
E) 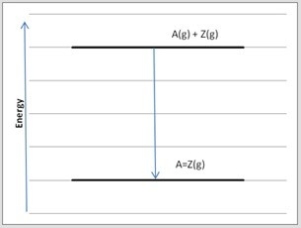
Correct Answer:

Verified
Correct Answer:
Verified
Q131: The standard temperature is _; the standard
Q132: A glass containing 200.0 g of H<sub>2</sub>O
Q133: A 307-g sample of an unknown mineral
Q134: A system contracts from an initial volume
Q135: Suppose a new metallic element X is
Q136: Given that CaO(s) + H<sub>2</sub>O(l) → Ca(OH)<sub>2</sub>(s),
Q137: For which of the substances below is
Q138: Pentaborane B<sub>5</sub>H<sub>9</sub>(s) burns vigorously in O<sub>2</sub> to
Q139: When heat is absorbed by the system
Q141: Which of the following has a standard