Multiple Choice
Which represents the result of mixing equal volumes of 1 M aluminum chloride, 2 M magnesium chloride, and 1 M potassium chloride solution? (Each sphere represents 1 mol of ions.)
A) 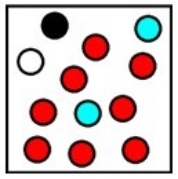
B) 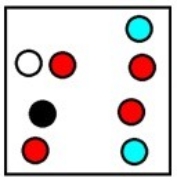
C) 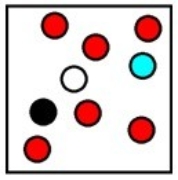
D) 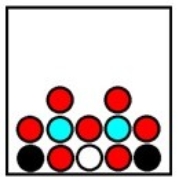
E) 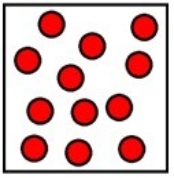
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q91: Suppose a large piece of a metallic
Q92: For the reaction depicted below, if Z
Q93: What is the oxidation number of N
Q94: What is the oxidizing agent in the
Q95: A standard solution of 0.243 M NaOH
Q97: Which chemical equation describes an acid-base neutralization
Q98: Which element is reduced in the following
Q99: Which of the following is insoluble in
Q100: 35.0 mL of 0.255 M nitric acid
Q101: _ is the name given for the