Multiple Choice
Suppose a large piece of a metallic solid, A(s) , is placed in a beaker containing the metallic cation Z2+(aq) , as depicted below. 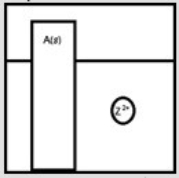 If Z is a more active metal than A, which is a possible representation of the final reaction mixture?
If Z is a more active metal than A, which is a possible representation of the final reaction mixture? 
A) I only
B) II only
C) III only
D) I or II
E) II or III
Correct Answer:

Verified
Correct Answer:
Verified
Q86: Which chemical equation describes a combustion reaction?<br>A)
Q88: What mass of lithium phosphate is needed
Q89: What is reduced in the following reaction?
Q90: What is the net ionic equation if
Q92: For the reaction depicted below, if Z
Q93: What is the oxidation number of N
Q94: What is the oxidizing agent in the
Q95: A standard solution of 0.243 M NaOH
Q96: Which represents the result of mixing equal
Q127: What is the pH of a 0.056