Multiple Choice
A 100.0-mL solution contains 27.8 g of sodium cyanide. Which type of solution is this? 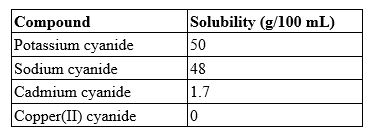
A) saturated
B) supersaturated
C) undersaturated
D) unsaturated
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: What is the pH of a 1.4
Q11: Which term is used to describe a
Q12: Which of the following compounds is soluble
Q13: What is the pH of a 0.280
Q14: What is the molarity of a solution
Q15: What ion would be used to form
Q16: Identify Ca(OH)<sub>2</sub> as a strong acid, strong
Q17: An unknown solution is determined to have
Q18: Which of the following compound is a
Q19: A 25.0-mL solution contains 0.45 g of