Multiple Choice
A sample of argon gas at 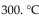 and 50.0 atm pressure is cooled in the same container to a temperature of
and 50.0 atm pressure is cooled in the same container to a temperature of 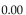
 What is the new pressure, if volume and amount of gas do not change?
What is the new pressure, if volume and amount of gas do not change?
A) 105 atm
B) 42.7 atm
C) 45.5 atm
D) 54.9 atm
E) 23.8 atm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: What unit of temperature is used in
Q19: A 5.00-L tank contains helium gas at
Q20: The relationship <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="The relationship
Q21: The unit of 1 atmosphere used to
Q22: As you rise higher in Earth's atmosphere,
Q24: The total pressure in a mixture of
Q25: At constant temperature, a sample of helium
Q26: According to the kinetic theory of gases,
Q27: According to the kinetic molecular theory of
Q28: A gas at 5.00 atm