Multiple Choice
A gas at 5.00 atm pressure was stored in a tank during the winter at 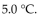 During the summer, the temperature in the storage area reached
During the summer, the temperature in the storage area reached 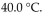 What was the pressure in the gas tank then, if volume and amount of gas do not change?
What was the pressure in the gas tank then, if volume and amount of gas do not change?
A) 69.5 atm
B) 5.63 atm
C) 40.0 atm
D) 0.625 atm
E) 4.44 atm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q23: A sample of argon gas at <img
Q24: The total pressure in a mixture of
Q25: At constant temperature, a sample of helium
Q26: According to the kinetic theory of gases,
Q27: According to the kinetic molecular theory of
Q29: Atmospheric pressure on a rainy day is
Q30: A tank contains helium gas at
Q31: A gas sample contains 4.0 g of
Q32: At STP, temperature and pressure have the
Q33: A 5.00-L tank contains helium gas at