Multiple Choice
A sample of nitrogen gas had a volume of 500 .mL, a pressure in its closed container of 740 torr, and a temperature of 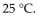 What was the new volume of the gas when the temperature was changed to
What was the new volume of the gas when the temperature was changed to 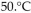 and the new pressure was 760 torr, if the amount of gas does not change?
and the new pressure was 760 torr, if the amount of gas does not change?
A) 400 mL
B) 240 mL
C) 450 mL
D) 970 mL
E) 530 mL
Correct Answer:

Verified
Correct Answer:
Verified
Q30: A tank contains helium gas at
Q31: A gas sample contains 4.0 g of
Q32: At STP, temperature and pressure have the
Q33: A 5.00-L tank contains helium gas at
Q34: Gay-Lussac's law is usually written as _.
Q37: How many moles of neon occupy a
Q38: According to Avogadro's law,<br>A)the volume of a
Q39: The volume of a gas with a
Q40: Which measurement describes the pressure of a
Q56: A barometer is usually filled with _.