Multiple Choice
A tank contains helium gas at 490 mmHg , nitrogen gas at 0.75 atm, and neon at 520 torr. What is the total pressure in atm?
A) 0.55 atm
B) 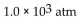
C) 1.5 atm
D) 2.1 atm
E) 1600 atm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q25: At constant temperature, a sample of helium
Q26: According to the kinetic theory of gases,
Q27: According to the kinetic molecular theory of
Q28: A gas at 5.00 atm
Q29: Atmospheric pressure on a rainy day is
Q31: A gas sample contains 4.0 g of
Q32: At STP, temperature and pressure have the
Q33: A 5.00-L tank contains helium gas at
Q34: Gay-Lussac's law is usually written as _.
Q35: A sample of nitrogen gas had a