Multiple Choice
The reaction of methane with oxygen to produce carbon dioxide and water is an example of which class of reaction? 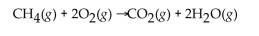
A) combustion
B) endothermic
C) single replacement
D) double replacement
E) combination
Correct Answer:

Verified
Correct Answer:
Verified
Q29: A chemical equation is balanced when<br>A)the total
Q30: In an oxidation-reduction reaction, the substance reduced
Q31: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="For the
Q32: A reaction that releases energy as it
Q33: What coefficient is placed in front of
Q35: In the following reaction, when the equation
Q37: How many moles of carbon are there
Q38: Pentane ( C<sub>5</sub>H<sub>12</sub> )reacts with oxygen
Q38: When 85.0 g of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg"
Q39: What is the classification for this reaction?