Multiple Choice
When 85.0 g of 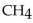 are mixed with 160 . g of
are mixed with 160 . g of 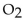 how many moles of
how many moles of 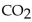 that can be produced?
that can be produced?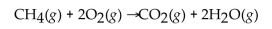
A) 2.50 moles
B) 7.81 moles
C) 5.00 moles
D) 5.31 moles
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: What coefficient is placed in front of
Q34: The reaction of methane with oxygen to
Q35: In the following reaction, when the equation
Q37: How many moles of carbon are there
Q38: Pentane ( C<sub>5</sub>H<sub>12</sub> )reacts with oxygen
Q39: What is the classification for this reaction?
Q40: How many moles of iron are present
Q41: 0.100 mole of lithium has a mass
Q42: What is the molar mass of copper(II)
Q43: The _ is the energy difference between