Multiple Choice
When 60.0 g of 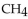 reacts with excess
reacts with excess 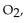 the actual yield of
the actual yield of 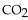 is 112 g. What is the percent yield?
is 112 g. What is the percent yield?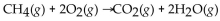
A) 67.9 %
B) 46.4 %
C) 187 %
D) 53.6 %
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q77: In the reaction of silver nitrate with
Q78: What type of reaction is the following?
Q79: How many grams of hydrogen are needed
Q80: In any balanced chemical equation, the number
Q81: What is the molar mass of sodium
Q83: For the question(s)that follow, consider the following
Q84: In an oxidation-reduction reaction, the substance oxidized
Q85: How many moles of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="How
Q86: For the question(s)that follow, consider the following
Q87: Find the mass of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="Find