Multiple Choice
Find the mass of 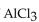 that is produced when 10.0 grams of
that is produced when 10.0 grams of 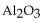 react with 10.0 g of HCl according to the following equation.
react with 10.0 g of HCl according to the following equation.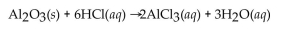
A) 10.0 g
B) 6.10 g
C) 16.2 g
D) 12.2 g
E) 20.0 g
Correct Answer:

Verified
Correct Answer:
Verified
Q82: When 60.0 g of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg"
Q83: For the question(s)that follow, consider the following
Q84: In an oxidation-reduction reaction, the substance oxidized
Q85: How many moles of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="How
Q86: For the question(s)that follow, consider the following
Q88: When 85.0 g of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg"
Q89: How many atoms of neon are present
Q90: 4.00 moles of sodium have a mass
Q91: 1.25 moles of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg"
Q92: What is the classification for this balanced