Multiple Choice
Barium chloride and sodium sulfate react according to the following equation. 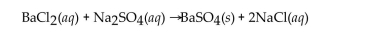 Answer the question(s) that follow about this reaction.
Answer the question(s) that follow about this reaction.
-How many moles of barium sulfate are produced from 0.100 mole of barium chloride?
A) 0.100 mole
B) 1.00 mole
C) 2.00 moles
D) 0.0100 mole
E) 0.200 mole
Correct Answer:

Verified
Correct Answer:
Verified
Q86: For the question(s)that follow, consider the following
Q87: Find the mass of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="Find
Q88: When 85.0 g of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg"
Q89: How many atoms of neon are present
Q90: 4.00 moles of sodium have a mass
Q91: 1.25 moles of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg"
Q92: What is the classification for this balanced
Q93: For the reaction: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="For the
Q94: What is the molar mass of NaBr?
Q96: In the following reaction, when the equation