Multiple Choice
The strongest interactions between molecules of ammonia, 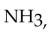 are
are
A) polar covalent.
B) ionic bonds.
C) dispersion forces.
D) dipole-dipole attractions.
E) hydrogen bonds.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: If the electronegativity difference between elements X
Q3: Choose the best Lewis structure for <img
Q4: The water molecule has a dipole with
Q5: Which of the following compounds contains an
Q6: The strongest interactions between molecules of hydrogen,
Q7: The types of compounds that use prefixes
Q8: What is the name of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg"
Q9: Valence electrons are electrons located<br>A)in the nucleus
Q10: Which of the following polyatomic ions has
Q11: In ionic compounds, _ lose their valence