Multiple Choice
What is the pH of a solution with 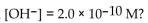
A) 9.70
B) -9.70
C) 4.30
D) 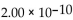
E) -4.30
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q47: How many milliliters of 0.400 M NaOH
Q48: Which solution has the lowest pH?<br>A)a buffer
Q49: According to the Brønsted-Lowry definition,<br>A)an acid acts
Q50: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8494/.jpg" alt=" " class="answers-bank-image d-block" rel="preload"
Q51: In a buffer system of HF and
Q53: How many milliliters of 0.200 M NaOH
Q54: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="The
Q55: Which of the following could be a
Q56: According to the Arrhenius concept, if NaOH
Q57: Ammonium hydroxide is a weak base because<br>A)it