Multiple Choice
In a buffer system of HF and its salt, NaF,
A) the HF neutralizes added acid.
B) the 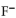 neutralizes added base.
neutralizes added base.
C) the HF neutralizes added base.
D) the 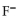 neutralizes added
neutralizes added 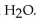
E) the HF is not necessary.
Correct Answer:

Verified
Correct Answer:
Verified
Q46: How many moles of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="How
Q47: How many milliliters of 0.400 M NaOH
Q48: Which solution has the lowest pH?<br>A)a buffer
Q49: According to the Brønsted-Lowry definition,<br>A)an acid acts
Q50: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8494/.jpg" alt=" " class="answers-bank-image d-block" rel="preload"
Q52: What is the pH of a
Q53: How many milliliters of 0.200 M NaOH
Q54: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="The
Q55: Which of the following could be a
Q56: According to the Arrhenius concept, if NaOH