Multiple Choice
A 25.0 mL sample of 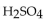 requires 20.0 mL of 2.00 M KOH for complete neutralization. What is the molarity of acid?
requires 20.0 mL of 2.00 M KOH for complete neutralization. What is the molarity of acid?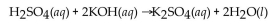
A) 0.800 M
B) 1.60 M
C) 2.00 M
D) 1.25 M
E) 2.50 M
Correct Answer:

Verified
Correct Answer:
Verified
Q62: In which of the following are the
Q63: How many milliliters of 0.100 M
Q64: The conjugate base of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="The
Q65: The name given to an aqueous solution
Q66: Which of the following statements correctly describes
Q67: What is the <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="What is
Q68: What is the pH of a
Q69: Which of the following is the strongest
Q70: What is the pH of a solution
Q71: What is the <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="What is