Multiple Choice
The molecular formula C2H4O can be converted into three-line bond (Kekulé) structures that are consistent with valence rules. Which one of the following Kekulé structures is not consistent with valence rules?
A) 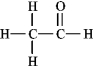
B) 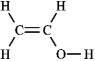
C) 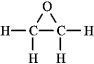
D) 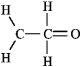
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: Instructions: Consider the reaction below to answer
Q9: Instructions: Consider the two structures below
Q10: Which is the strongest base (pK<sub>a</sub> values
Q12: Instructions: Use the convention <span
Q14: Use the curved arrow method to show
Q15: Which of the following statements is not
Q16: Consider the structure of acetic acid shown
Q17: Instructions: Consider the two structures below
Q18: Instructions: Indole is pleasant smelling in highly
Q27: In drawing the Lewis structure for an