Multiple Choice
Consider the structure of acetic acid shown below. 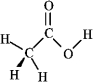 In the electrostatic potential map of acetic acid, in which of the following bonds would the terminal atom appear as the deepest shade of red?
In the electrostatic potential map of acetic acid, in which of the following bonds would the terminal atom appear as the deepest shade of red?
A) C=O
B) C-H
C) C-C
D) O-H
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: Instructions: Use the convention <span
Q13: The molecular formula C<sub>2</sub>H<sub>4</sub>O can be converted
Q14: Use the curved arrow method to show
Q15: Which of the following statements is not
Q17: Instructions: Consider the two structures below
Q18: Instructions: Indole is pleasant smelling in highly
Q19: The following shows an intermediate used in
Q20: Instructions: Label the acid and base in
Q21: Draw a Lewis structure for each of
Q27: In drawing the Lewis structure for an