Multiple Choice
One of the main problems with the Bohr model of the hydrogen atom when compared with the results of the methods of quantum mechanics used to describe atoms, was that the Bohr model predicted:
A) the ground state angular momentum was L = 1 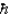
B) the frequency of the radiation emitted when an electron 'jumps' from one allowed orbit to another was hf = Ei - Ef.
C) the potential energy function for the hydrogen atom was given by V(r) = -ke2/r.
D) the energy of the ground state of the hydrogen atom was En =-13.6 eV.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: When electrons fill a subshell in which
Q15: The Pauli Exclusion Principle states<br>A) no two
Q26: The energy difference between the upper and
Q39: The ground state configuration of chlorine (Z
Q42: All quantum states forming a shell have
Q43: What angle does the orbital angular
Q45: In an allowed electron transition in
Q46: What is the difference in frequency for
Q47: Adam and Eve are contemplating the
Q49: An energy of 13.6 eV is needed