Multiple Choice
In an allowed electron transition in a hydrogen atom:
A) ( 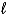 = 0;
= 0; 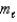 = 0, 1. )
= 0, 1. )
B) ( 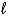 = 0, 1;
= 0, 1;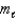 = 1. )
= 1. )
C) ( 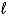 = 0, 1;
= 0, 1; 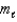 = 0, 1. )
= 0, 1. )
D) (  = 1;
= 1; 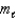 = 0, 1. )
= 0, 1. )
E) ( 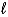 = 1;
= 1; 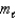 = 1. )
= 1. )
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: When electrons fill a subshell in which
Q15: The Pauli Exclusion Principle states<br>A) no two
Q26: The energy difference between the upper and
Q39: The ground state configuration of chlorine (Z
Q42: All quantum states forming a shell have
Q43: What angle does the orbital angular
Q46: What is the difference in frequency for
Q47: Adam and Eve are contemplating the
Q48: One of the main problems with the
Q49: An energy of 13.6 eV is needed