Multiple Choice
In a shell of the hydrogen atom with n = 3, the permitted values of the orbital magnetic quantum number are: 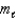
A) (-1, 0, 1)
B) 2, 1, 0
C) 2, 1, 0, -1, -2
D) 0
E) 3, 2, 1, 0, -1, -2, -3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: In an atom that has an electron
Q12: A hydrogen atom is in its first
Q13: When using the Pauli Exclusion Principle,
Q15: All quantum states forming a sub-shell have
Q17: The radial portion of the de
Q18: The magnitude of the spin angular
Q19: For the following allowed transitions, which photon
Q39: Suppose a beam of electrons is incident
Q42: Aline says that the magnetic moment of
Q54: Rubidium (Z = 37) and potassium (Z